INDIAN ARMED FORCES CHIEFS ON OUR RELENTLESS AND FOCUSED PUBLISHING EFFORTS

The insightful articles, inspiring narrations and analytical perspectives presented by the Editorial Team, establish an alluring connect with the reader. My compliments and best wishes to SP Guide Publications.

"Over the past 60 years, the growth of SP Guide Publications has mirrored the rising stature of Indian Navy. Its well-researched and informative magazines on Defence and Aerospace sector have served to shape an educated opinion of our military personnel, policy makers and the public alike. I wish SP's Publication team continued success, fair winds and following seas in all future endeavour!"

Since, its inception in 1964, SP Guide Publications has consistently demonstrated commitment to high-quality journalism in the aerospace and defence sectors, earning a well-deserved reputation as Asia's largest media house in this domain. I wish SP Guide Publications continued success in its pursuit of excellence.
- MoD initiates comprehensive review of Defence Acquisition Procedure 2020, pushes for defence reforms
- G7: The Swansong
- Kalinga Connect: South Asia to Polynesia
- Advanced MRSAM for India for a greater firepower
- Must Credit DRDO for Operation Sindoor, now what is next for defence R&D?
- Operation Sindoor | Day 2 DGMOs Briefing
- Operation Sindoor: Resolute yet Restrained
2-DG Drug Helping COVID-19 Patients
DRDO affiliated Institute of Nuclear Medicine and Allied Sciences (INMAS) worked in partnership with Dr Reddy’s Laboratories for the clinical trials showing it to be effective in controlling the pandemic
 |
The Author is Former Director General of Information Systems and A Special Forces Veteran, Indian Army |

On May 17, 2021, Defence Minister Rajnath Singh along with the Union Health Minister Harsh Vardhan officially released the 2-DG drug in a ceremony. Earlier on May 1, the Drug Controller General of India (DCGI) and the Central Drugs Standard Control Organization (CDSCO) gave permission for the emergency use of drug 2-deoxy-D-glucose (2-DG) manufactured by Dr Reddy Labs as an adjunct therapy in moderate to severe Covid-19 cases. 2-DG promises to reduce hospitalisation time and oxygen dependency for moderate to severe cases. DRDO affiliated Institute of Nuclear Medicine and Allied Sciences (INMAS) played a significant role to facilitate the trials and helping to get the DGCI-CDSCO sanctions.
Feedback of the drug on social media is good stating that it provides relief to patients with low oxygen levels
The stated mission of INMAS is clinical research in nuclear medicine and non-invasive imaging methods with a focus on biological radio-protectors and thyroid disorders. INMAS has established the Nuclear Magnetic Resonance (NMR), PET-Cyclotron facility for enhancing combat efficiency and clinical research for the Armed forces. The institute also has a research programme with application in various stress-related disorders using NMR techniques including metabolomics, in vivo spectroscopy and functional MRI (fMRI).

Clicking on ‘Organization’ and ‘Products’ on INMAS website draws blanks perhaps to hide the size of its organisation (perhaps elephantine like other DRDO entities) and how many products it has produced since its establishment in 1956. However, its main contribution to Armed Forces has been diagnosis, prevention and treatment of cold injuries (frostbite) for troops deployed in high altitude areas. INMAS website however also claims establishing several non-invasive and innovative nuclear medicine methods, and that INMAS is now working in biological radioprotection (radiation countermeasures); management of thyroid disorders; nuclear and medical imaging; combat casualty management; CBRNe medical management.
The contribution by Dr Reddy’s Lab is the major one although 2-DG is being portrayed as a DRDO triumph
INMAS worked in partnership with Dr Reddy’s Laboratories for the clinical trials showing it to be effective in controlling the pandemic. As mentioned above, 2-DG was manufactured by Dr Reddy Labs but this fact was kept ambiguous by design in media reports so that DRDO could claim the success. But, Dr Sudhir Chandna of DRDO said in a video that Dr Reddy’s Lab will come out with the pricing and roll out the 2-DG drug in a few days. This showed that the contribution by Dr Reddy’s Lab is the major one although 2-DG is being portrayed as a DRDO triumph. But that is the DRDO way. The drug comes in powder form in a sachet and is to be taken orally by dissolving it in water. Feedback of the drug on social media is good stating that it provides relief to patients with low oxygen levels.
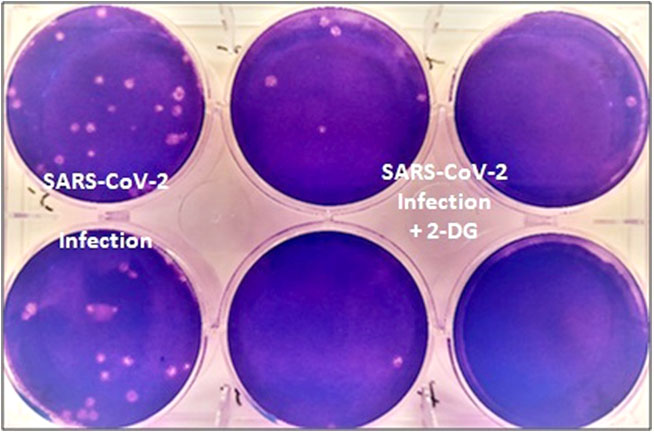
Recall how highly advanced technology bullet proof jackets (BPJs) developed by Amrita University, Coimbatore and their proposal titled “Development of Hyper Velocity Impact Resistant Ultra Light Weight High Temperature Resistant Thermoplastic Polymer-Carbon Fiber Composite” kept lying with MoD till DRDO acted the go-between to get government approval, claiming that to be joint effort. But DRDO enjoys politico-bureaucratic patronage being the goose that lays golden eggs. With regard to the 2-DG drug, DRDO issued a statement saying, “Being a generic molecule and analogue of glucose, it (2-DG) can be easily produced and made available in plenty."
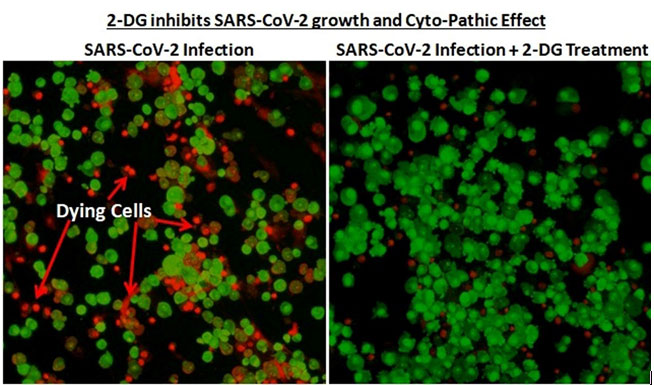
Work on the drug started in April 2020 during the first wave of the pandemic, with scientists identifying its therapeutic abilities, following which the Drugs Controller General of India permitted clinical trials for 2-DG. The phase-2 trials were conducted with 110 patients at 11 hospitals, while phase 3 was conducted on 220 patients across 27 hospitals, concluding in March this year. According to officials, “A significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence by day 3, indicating an early relief from oxygen therapy/dependence. A similar trend was observed in patients more than 65 years old.”
Indian Space Research Organisation (ISRO) has developed three different types of ventilators and an oxygen concentrator
DRDO Chief G. Satheesh Reddy told media on May 8, “In the next one week, we will have 10,000 doses ready and there will be a large rollout in the next three weeks. This will help support patients and prevent the spread of the virus. We are working closely with Dr Reddy’s Laboratories to ramp up availability,” This is an excellent development that will help India fight the pandemic, help to ease the pressure on hospitals and help the Covid-19 patients recover faster. Government needs to ensure its availability across the country in an early timeframe.
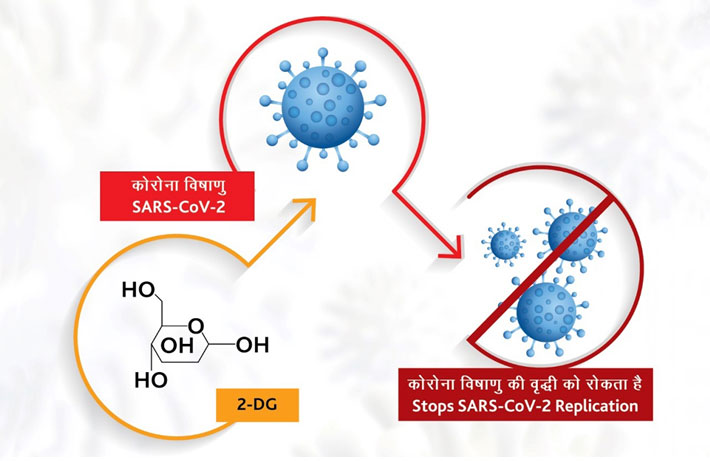
A second encouraging news is that the Vikram Sarabhai Space Centre (VSSC) of the Indian Space Research Organisation (ISRO) has developed three different types of ventilators and an oxygen concentrator at a time when a shortage of these critical medical equipment resulted in deaths of many Covid-19 patients across the country. The VSSC started working on these ventilators at the beginning of the first wave of Covid-19 in March 2020 but the work slowed down with the threat diminishing late last year. However, the institute was asked to expedite work after the second wave of the pandemic stunned the country’s healthcare system, causing several deaths due to lack of ventilators, oxygen and Covid-19 medicines like the Remdesivir.
S. Somanath, Director of VSSC told media, “Based on designs, features and specifications, we have named them, ‘Prana’, ‘VaU’ and ‘Svasta’. All three are user-friendly, fully automated and with touch-screen specifications, meeting all safety standards”, adding that doctors and other experts have checked its efficacy and confirmed it meets international standards. The plan is to undertake technology transfer for the commercial production of the three ventilators and the one oxygen concentrator within May 2021. These VSSC developed are likely to be priced at one lakh per unit and are easy to handle making them cost effective compared to the mini conventional ventilators that are currently priced around five lakh apiece.
The above are no doubt good developments but these should not make us sit back and gloat. Reliance has recently imported a Covid testing machine from Israel which gives the result in one minute. We certainly need wide ranging research and development to combat the pandemic.





